Medtronic launched new devices for the treatment of heart failure
Recently the world-famous Canadian company Medtronic has introduced a new product on the market — the Amplia MRI Quad CRT-D Sure Scan. Due to this novelty, patients with heart failure can safely undergo MRI procedures for the first time. Before it seemed almost impossible to solve this issue,however due to the use of modern technologies, the efforts of leading clinicians and scientists, the device was licensed by the Canadian government and entered the market.
Nowadays, heart failure is one of the most common diseases, the treatment of which requires considerable effort. Modern medicine does its best to make heart failure treatment methods more effective and a new Medtronic device undoubtedly proves this. The machine was developed by the subsidiary of Medtronic and made it possible to conduct magnetic resonance imaging for people with pacemakers.
Device Features
It has been known for a long time that diagnostic procedures of magnetic resonance imaging are contraindicated in people with implanted metal devices, however they might need to undergo MRI.
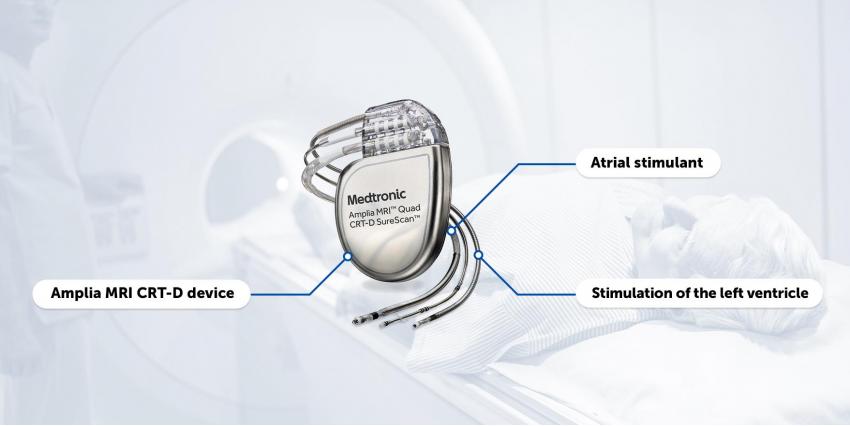
Until now, ultrasound or CT have been used as an alternative to MRI. In 2011, Medtronic obtained a license from the Canadian government for the development of Amplia MRI Quad CRT-D Sure Scan and Compia MRI Quad CRT-D Sure Scan. Consequently, now patients with internal implants will be able to undergo MRI procedure safely. What is more, new devices can be used to examine all parts of the body, without exception.
To use the device for MRI, Medtronic needs a complete SureScan CRT-D system, which includes the following components:
- Amplia MRI CRT-D device;
- Sure Scan atrial stimulant or plug of 6725 model for right atrial port;
- Sure Scan for stimulation of the left ventricle.
Device Operation Mode

The novelty has entered the market of medical products and created new opportunities for the treatment of heart diseases. The Amplia MRI CRT-D system works on the basis of the Adaptiv CRT algorithm, which minimizes the risk of patient’s hospital readmission, accelerates the CRT reaction up to 12%, and reduces the probability of atrial fibrillation. The device also provides multi-point stimulation, which can simultaneously stimulate two areas in the left ventricle (lower heart chamber).
The director of Medtronic corporation, Michael Blackwell, claims that this invention creates new opportunities for doctors dealing with patients with heart failure. The system is used to stimulate the ventricles, as well as to solve the problem with ventricular arrhythmia, that threatens lives.

Patients can be scanned using a horizontal field, a cylindrical bore, a 1.5T or 3T MRI clinical system, as a result, hydrogen proton images are received with a maximum spatial gradient which is ≤ 20 T / m and a maximum gradient rate per axis which is ≤ 200 T / m / s.
During MRI procedures, patients with implants are supposed to follow such requirements:
- the absence of lead extension cords and adapters;
- the absence of broken wires or wires with intermittent contact;
- the stimulation threshold should not exceed 2 V.

An incorrect application of the system can lead to undesirable consequences and health complications after the procedure. That is why, the review of the technical specifications of the device showed that only professionals in the field of cardiology can work with such system.
Medtronic is the world's leading company, developing innovative medical technologies for the treatment of cardiovascular diseases. On top of that, it offers both effective innovations and affordable prices for medical institutions.
21.11.2017